Genetic defect enlarges endothelial cells and causes vessel malformations
Cell size rather than number crucial for occurrence of Osler-Weber-Rendu disease
Through a complex vascular system, the heart supplies even the most remote regions of our body with blood. The correct size of each vessel is for the most part regulated according to blood pressure and shear stress. A team of scientists led by Arndt Siekmann from the Max Planck Institute for Molecular Biomedicine in Münster has studied how haemodynamic forces in the vessels influence vessel patterning. The scientists were able to identify in zebrafish one of two genes that can cause hereditary haemorrhagic telangiectasia, also known as Osler’s disease, in humans. The new results show that enlarged endothelial cells in blood vessels can give rise to malformations in the vascular network, because they cannot react to the incipient blood flow during embryonic development by correctly altering their shape. The team of researchers was also able to demonstrate that the size of blood vessels is largely determined by the shape, not just the number, of the endothelial cells that form their lining. Blood flow measurements performed by Cornelia Denz of the Institute of Applied Physics of the University of Münster allowed the team to develop a new model that elucidates the role of correct endothelial cell shape and size in blood vessel development and in the emergence of vessel malformations (Nature Cell Biology, advance online publication, May 22, 2017).
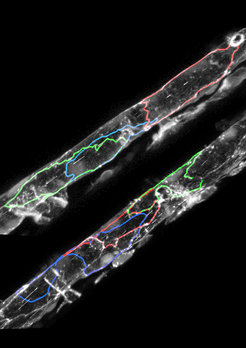
Blood vessels of normal (top) and endoglin-deficient zebrafish (bottom) with individual endothelial cell outlines labelled. Abnormal cell organization leads to larger vessels in endoglin mutants.
The vascular system is hierarchically structured. Larger vessels lead to different organs, in which the blood vessels branch off into ever-smaller parts. The tiniest blood vessels, the capillaries, are where the exchange of nutrients and waste takes place. Vascular malformations will cause an insufficient blood supply to the tissue, and often also haemorrhaging, with potentially serious health consequences. Arteriovenous malformations (AVM) are characterized by direct connections between larger arteries and veins, bypassing the intervening capillary bed. The alk1 and endoglin genes are involved in AVM development, but the exact mechanism behind this type of deformity was hitherto unknown.
In order to understand the processes leading to AVMs, Arndt Siekmann and his colleagues from the Max Planck Institute in Münster studied the vascular system of zebrafish embryos. “The great advantage of zebrafish embryos is that they are transparent, which means that we can see inside the embryo”, Siekmann says. The scientists can stain cells in the embryonic blood vessels using fluorescent proteins and even measure the shape and size of endothelial cells. Once the scientists had deactivated the endoglin gene in zebrafish embryos, they observed that endothelial cells carrying the mutated endoglin gene were larger than in normal animals. “Previously, it was widely assumed that enlarged blood vessels were caused by an increase in the number of endothelial cells in affected vessels”, says Wade Sugden, first author of the study and PhD student in the graduate school of the Cells-in-Motion Cluster of Excellence and the Max Planck Institute.
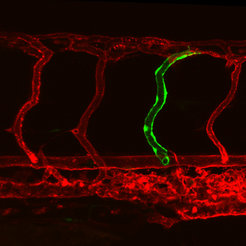
After transplanting some cells from a endoglin-mutant zebrafish embryo, a dilated blood vessel (green) emerged within the blood vessel network (red) of a healthy zebrafish.
When the blood flow begins during early embryonic development, the blood vessels expand; however, they subsequently narrow again. Sugden explains that this is because the endothelial cells become elongated and rearrange themselves along the vessel. “The realisation that it is the altered shape of the endothelial cells during early embryonic development that influences vessel size is new”, Siekmann says. “Up to now, scientists believed that it was mainly the surrounding smooth muscles that determined the circumference of the vessels.” The adjustment of the vessel size causes the blood flow to be distributed evenly to all capillaries in the vascular network. In endoglin deficient zebrafish, the situation is markedly different: “The larger endothelial cells do not arrange themselves correctly when the blood begins to flow – the vessels remain too wide”, Sugden says. “Consequently, less blood runs through the other capillaries, which remain smaller. Instead of a harmoniously formed vessel tree, there is disequilibrium between too large and too small blood vessels.” This type of deformity therefore occurs in the early developmental stages of the vascular network.
Abnormal blood flow by endoglin-malfunction
In normal zebrafish embryos (top) blood flow in the major artery (upper vessel) is pulsatile, in the major vein (lower vessel) smooth and continuous. Red blood cells pass through the connecting vessels.
In blood vessels of endoglin-deficient zebrafish embryos (bottom), the blood flow velocity is pathologically high. No red blood cells pass through the connecting blood vessels.
Still, the subsize capillaries are principally functional. Using optical tweezers, Cornalia Denz and colleagues from the University of Münster employed the force of light to reduce the blood flow rate in expanded vessels. As a result, the slower red blood cells started to dart down the previously unperfused capillaries. “This functional test confirms the new understanding of the role of endoglin in vessel formation”, Siekmann says. The current study therefore sheds new light on how arteriovenous malformations occur. “Future treatments of hereditary haemorrhagic telangiectasia could aim to normalise endothelial cell size”, Siekmann says.

