Nuclear architecture diagnostics within reach of the clinic
A new technique that allows the study of the 3D architecture of cancer genomes might help in the design of personalized treatment strategies
Scientists at the Max Planck Institute of Molecular Biomedicine in Münster and of the Medical Faculty of the University of Münster have developed a technique that allows the characterisation of the three-dimensional organisation of the DNA in the nucleus directly in patient’s cells. The research, published in Nature Communications (online November 29, 2018), will help in diagnosing disease and, in future, guiding therapeutic intervention.
Each cell in our organism has a roughly 2-meter-long molecule of DNA – our genetic information – that needs to be properly packed inside a few micron nucleus. How the DNA is organised in the nucleus is known to play a key role for normal cellular development and function, since mutations in the mechanisms that control this process lead to developmental disorders or diseases such as cancer. However, the exact role that the organisation of the genome plays in disease is currently unknown, since scientists have lacked the ability to thoroughly examine the 3D organisation of the genome in diseased cells.
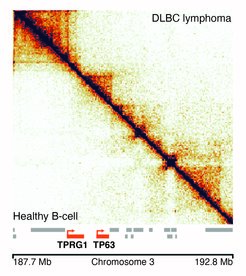
In this new research, scientists have performed a proof-of-principle study demonstrating that the 3D genome can be directly examined in diseased cells from patients. Subtle improvements implemented in this new technique to measure three-dimensional genome architecture, called Low-C, allowed researchers to lower the amount of biological material initially required to perform the experiments. This enabled them to determine the spatial architecture of a diffuse large B-cell lymphoma genome. “To be able to examine the genome architecture of the specific cells that cause disease is really exciting, since currently we do not know how the 3D genome is altered in these cells”, says Dr Noelia Díaz, a postdoctoral fellow in the Vaquerizas Laboratory who led the experimental part of the project.
The researchers then performed an advanced computational analysis of the data that revealed some surprising observations. First, the scientists were able to detect genome rearrangements – changes in the normal sequence arrangement of our genome that are a key feature of many cancers – and detected both novel and known translocations characteristic of the disease, which were then experimentally validated. “It was reassuring to see our computational predictions validated experimentally”, says Dr Kai Kruse, a postdoctoral fellow in the Vaquerizas Laboratory who performed the computational analysis of the data.
More power to study cancer cells
But the data held more surprises. When the researchers examined a finer level of chromatin organisation into topological domains (short sections of the genome that are folded into compact knots resembling balls of yarn), they observed that new domains were present in disease cells in regions of the genome that would otherwise present no domains in healthy cells. “This was a surprising finding, since the 3D architecture of fully developed cells is thought to be rather invariant”, says Dr Juanma Vaquerizas, a Group Leader at the Max Planck Institute for Molecular Biomedicine in Muenster, who supervised the research. “We could observe that these new structural domains appear in regions that contain genes previously known to be associated with cancer and disease, but the functional role of these new domains is currently unknown”, says Vaquerizas.
The researchers aim now to extend their studies to more samples, to be able to determine the impact that changes in the 3D structure of the genome play in disease and to use this information in the design of personalised patient-specific treatment options.
