Newly discovered blood vessel type in bone is responsible for bone-formation
Max Planck scientists: clinical implications for disorders of bone formation
Bone formation is essential for growth processes and fracture healing. To a lesser extent, the skeletal system of healthy adults is also renewed constantly. However, bone mass is gradually lost in the ageing organism or in diseases such as osteoporosis. The directed generation of new blood vessels in bone might counteract these negative effects. Yet the characterization of bone is technically difficult and, accordingly, very little is known about the exact mechanisms coupling bone growth and blood vessel formation. A team of scientists around Ralf H. Adams of the Max Planck Institute for Molecular Biomedicine has now overcome these difficulties and thereby discovered a new type of blood vessel in the skeletal system that is responsible for bone formation (Nature, online first, March 12, 2014; doi:10.1038/nature13145). The scientists have also identified signaling pathways that influence the formation of new blood vessels and thereby bone formation (Nature, online first, March 12, 2014; doi:10.1038/nature13146). These findings could facilitate the development of future therapies aiming at improved fracture healing and the prevention of bone loss.
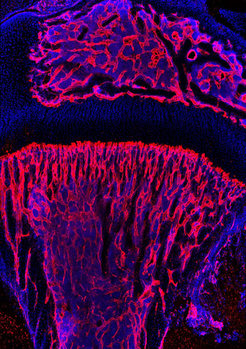
Blood vessels enable not only the transport of oxygen, nutrients and waste products, but they also provide signals that play important roles in organ growth and homeostasis. Endothelial cells form the inner lining of all blood vessels and have different functional roles, which strongly depend on their organ environment. Scientists have suspected for a long time that molecular connections link endothelial cells and bone-forming cells. Because blood vessels and bone-forming cells were often observed in close vicinity, it has been proposed that angiogenesis (the formation of new blood vessels) might promote fracture healing. The exact crosstalk between vessels and bone growth, and the organization of the blood vessel network in the skeletal system had remained largely unknown.
This knowledge gap has a technical background: bone is very hard on the outside, but on the inside, where the bone marrow sits, it is soft. If one wishes to investigate bone in the microscope, thin sections are made that require decalcification. However, while the material gets soft, the structure of the tissue along with the correct position of the blood vessels can be obscured. Immunohistological staining of specific markers in bone is also highly challenging.
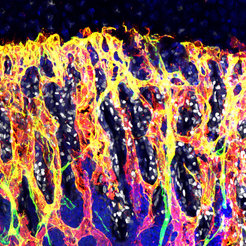
Professor Dr. Ralf Adams, who also is a professor of the Medical Faculty of the Westphalian Wilhelms-University Münster, and two of his colleagues, Dr. Anjali Kusumbe and Dr. Saravana Ramasamy, have overcome the technical hurdles by modifying the preparation procedure and by using a new high resolution 3D imaging technique. Kusumbe and Ramasamy have created transgenic mice whose blood vessels are marked in green in a fluorescence microscope. The tibia and the femur were the model bones in the study.
Corresponding to the various regions in the bone, the scientists found two different types of blood vessel, which they called type H and type L depending on the amount of marker proteins CD31 and endomucin (H for ‘high’ and L for ‘low’). Anjali Kusumbe explains: “In the growth plate of the bone, we exclusively discovered long type H capillaries and in the inner bone we found only capillaries of the L-type.” She continues: “Remarkably, bone-forming cells were preferentially situated in direct proximity of H-type capillaries.” This is a clear indication that H-type capillaries represent the connection between angiogenesis and osteogenesis (formation of new bone).
Now, the scientists got into the bottom of the molecular mechanisms. They encountered two signaling pathways that play an important role in the regulation of blood vessels: HIF (hypoxia-inducible factor) and Notch. The scientists already knew Notch as an angiogenic regulator in the retina. However, in the bone, Notch controls angiogenesis differently: “Against our expectations, we observed that Notch promotes angiogenesis in bone instead of suppressing it,” says Saravana Ramasamy. And also endothelial HIF is positively regulating angiogenesis and osteogenesis.
The scientists used this knowledge to interfere with these signaling pathways genetically and pharmacologically in order to control bone formation: in bone of mice that cannot produce HIF in endothelial cells, no H-type capillaries were found. In bone of mice with enhanced HIF activity, the scientists found more type H capillaries and, accordingly, more bone was formed.
Also the pattern in which new blood vessels arise in the bone is different from that in other organs: “The formation of new blood vessels is not initiated by so called ‘tip cells’ as we had observed earlier in the retina, but by endothelial cells that form tubular loops”, says Ramasamy.
From humans we know: bone formation is declining with age, which is why bone quality and fracture healing are deteriorating. At the same time, older people are more vulnerable to skeletal fractures. Kusumbe and Ramasamy therefore also had a look at the endothelial cell types in old mice. And indeed: “In old mice, we observed only very few H-type capillaries. At the same time, we saw less bone-forming cells and a reduction of bone mass,” says Kusumbe.
By using modified and new methods, a team of scientists around Ralf Adams has been able to close a decade-long knowledge gap. The scientists have not only demonstrated that blood vessel formation in bone is fundamentally different – they have also discovered that there are two different types of capillaries with specialized functions. One of the newly identified capillary types is responsible for bone formation. “These findings are of great importance for the development of future therapies for fracture healing and bone loss, as they occur during aging and osteoporosis”, says Ralf Adams. “If we can support the formation of H-type capillaries pharmacologically in patients, we could probably strengthen skeletal bones. Even a small, 10-percent increase in bone mass would already noticeably improve the situation in osteoporosis,” says Adams.

