A balancing act: EphB4 and ephrin-B2 regulate artery formation
Low EphB4 and high ephrin-B2 levels activate the arterial programme in the vascular cells
Conditions affecting the arterial vasculature, including myocardial infarction, stroke and coronary artery disease, present pressing challenges in global health. Yet, despite strides in medical science, the complex mechanisms underlying artery formation remain elusive, impeding the development of novel treatment modalities. A team of researchers from the Max Planck Institute for Molecular Biomedicine, led by Mara Pitulescu and Ralf Adams, have uncovered new insights into how arteries form. Building on their earlier discovery on the role of Notch-signaling pathway in directing "tip" cells to become arteries, their latest study published in Nature Communications reveals the crucial role of EphB4 and ephrin-B2 in shaping the fate of tip cells, controlling artery formation. These findings offer hope for novel treatments in cardiovascular medicine.
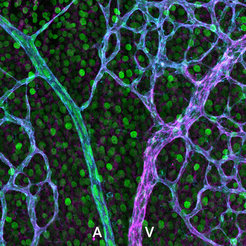
Nuclear genetic reporter highlighting ephrin-B2 (green) expression in arteries, while immunostaining shows EphB4 (magenta) dominating in veins of the retinal vasculature (cyan). A – Artery; V – Vein.
At the heart of this discovery lie two key molecules: EphB4 and ephrin-B2. Traditionally, EphB4, a transmembrane receptor protein, is linked to endothelial cells of the venous system, while ephrin-B2, a transmembrane ligand protein, dominates in arterial endothelial cells. Interestingly, recent studies have unveiled their co-expression in tip cells, the frontier navigators of vascular expansion. "Imagine the developing body's vascular system as an expanding network of roads, with arteries acting as life-sustaining highways for oxygen and nutrients. At the network's edge are tip cells, mediating expansion into new territories," explains Mara Pitulescu, senior scientist in the department of Ralf Adams. Pitulescu's earlier research showed the remarkable ability of tip cells to shift towards the arterial fate. Migrating against blood flow, these cells serve as building blocks that incorporate into arteries, mediating their growth.
In search of the molecular mechanisms underlying the fate specification of tip cells, Mara Pitulescu and PhD student Jonas Stewen analyzed the retinal vasculature of mouse mutants as well as cell culture models where EphB4 or ephrin-B2 signaling is impaired or enhanced. “We found that EphB4 and ephrin-B2 inhibit each other: When EphB4 is low and/or ephrin-B2 is high, the arterial program in endothelial cells is switched on,” Stewen says. Additionally, the researchers found intricate feedback loops from EphB4 and ephrin-B2 activating or inhibiting Notch and vascular endothelial growth factor (VEGF) signaling, known players involved in arterial fate acquisition. “The tight balance of EphB4 and ephrin-B2 thus orchestrates a well-wired molecular network governing arterial fate specification,” Stewen adds.

Genetically labelled tip cells (green) in the retinal vasculature (magenta). Arterial incorporation of tip cell progeny is increased in EphB4 mutants (right) compared to control mice (left). Arteriovenous crossings occurs in the vasculature of EphB4 mutants (arrowhead). A – Artery; V – Vein.
Strikingly, disrupting the balance of EphB4 and ephrin-B2 in tip cells has profound effects on whole vascular plexus development. “We found that deletion of EphB4 causes so-called arteriovenous crossings, a retinal vascular pathology found in human patients where an artery lies on top of the vein,“ Pitulescu says. This can lead to arteriovenous nicking and further retinal vein occlusion – a phenomenon increasingly linked to heightened risks of stroke formation.
"Our findings could shed light on the underlying mechanisms of certain vascular diseases, potentially paving the way for the development of novel therapeutic interventions," explains Ralf Adams, head of the Tissue Morphogenesis department at the Max Planck Institute for Molecular Biomedicine.

